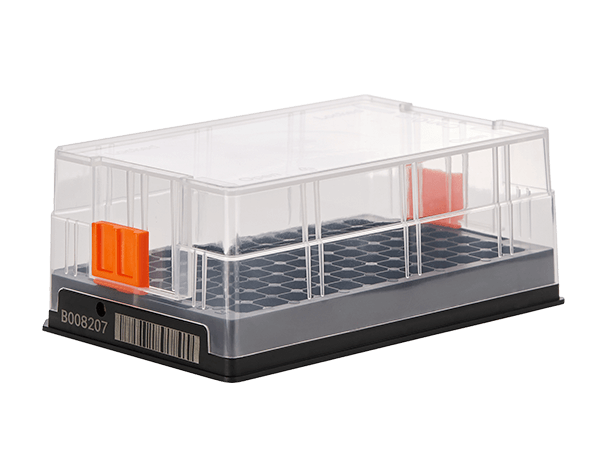
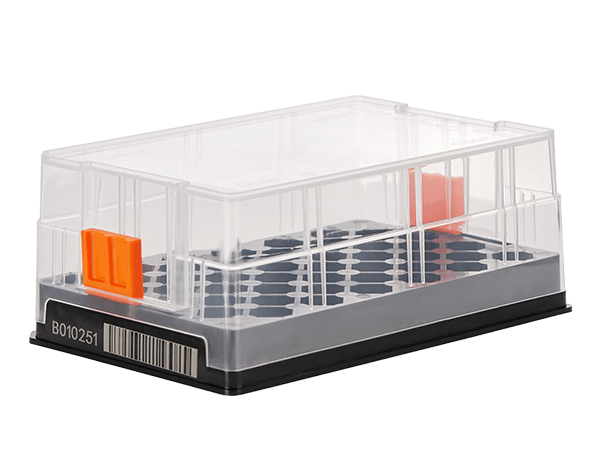
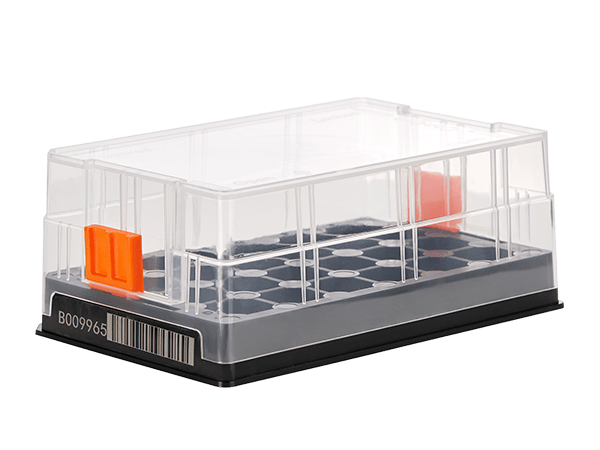
Bann SBS Cryobox fully considers the needs of users when managing complex samples, and all cryoboxes adopt highly consistent standardized SBS specification design;
Even if the user has customized the freezing rack according to the 9×9 and 10×10 Cryoboxes, the Bann SBS Cryobox can be accommodated by simply changing the drawer, and the overall capacity will not be affected;
Users only need to use one specification of freezing rack to meet the needs of different types of sample collection and preservation.
One dimensional code and digital code are marked on the side wall of Bann SBS CRYOBOX, and two-dimensional code is marked at the bottom of the CRYOBOX;
Sidewall one-dimensional code and digital code can be used for general identification;
The bottom QR code can be adapted to the Bann batch decoder. While decoding the tube code and location information, the Cryobox code is obtained, and the Cryobox code is associated with the tube code, which improves the efficiency of sample management.
Bann SBS Cryoboxes are all designed with standard SBS spacing. The arrangement of tubes of various specifications fully complies with the requirements of SBS specifications and standards, which is very suitable for the operation of automation equipment.
The Twist Lock is fixed to prevent the tubes body from falling and rotating in the box, which is convenient for automatic equipment to remove and seal the cover automatically.
It is specially used to hold the Cryobox of SBS specification.
Make the maximized space of refrigerators of different brands in the market.
The unified standard Cryobox is perfectly matched with the freezing rack, which is convenient for long-term use. There is no dilemma that the size of the newly purchased freezing box is not suitable for the freezing rack, which is not only convenient for storage, but also reduces the replacement cost.
ISO grade 8 clean workshop and automatic production line ensure that the products do not contain DNA / RNA / RNase / DNase / endotoxin / heat source.
Transportation safety: Accord with IATA requirements.
Product certification: EU CE certification (IVDD directive)
Production quality system: ISO13485 (2016 medical device quality management system certification)